H3C-C-C-OH H3C-C-C -O H3C-C-c-OH H3C C-C- H3C-C-C- H3C-C HO-. You will require some thing to produce modest dots with.

Biochemistry Final Exam Part 2 Everything Else Flashcards Practice Test Quizlet
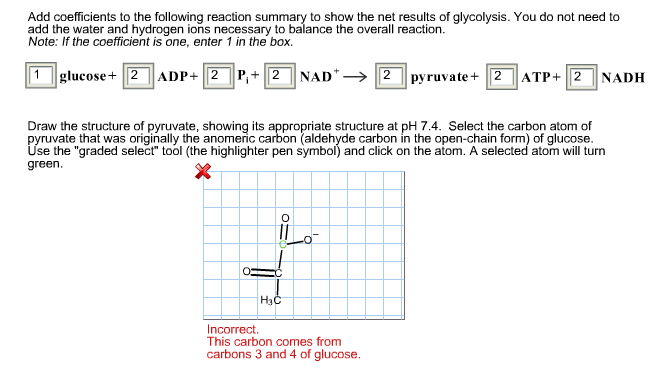
Select the carbon atom of pyruvate that was originally the anomeric carbon aldehyde carbon in the open-chain form of glucose.

. The structure should have a carbon highlighted in green and bold that corresponds to the original anomeric carbon aldehyde carbon in the openchain form of glucose. Then prime it off with a yellow dot at the middle. Draw the structure of melamine cyanurate which forms when melamine and cyanuric acid form hydrogen bonds with each other.
At pH 74 the optimum activity of pyruvate dehydrogenase E1 dihydrolipoamide acetyltransferase E2 and dihydrolipoamide dehydrogenase E3 occur in the ranges of ionic strengths of 006-008 001-002 and 010. Up to 256 cash back If no coefficient is needed leave the answer blank. Pyruvate C3H3O3- CID 107735 - structure chemical names physical and chemical properties classification patents literature biological activities safety.
165000 C at 760 mmHg. PHpKalog AHA Pyruvic acid. A representative plot showing the change in absorbance at 220 nm versus the time after mixing a solution of sodium pyruvate 075 mM with H 2 O 2 10 mM in 40 mM buffer phosphate pH 74 ionic strength 015 at 25C.
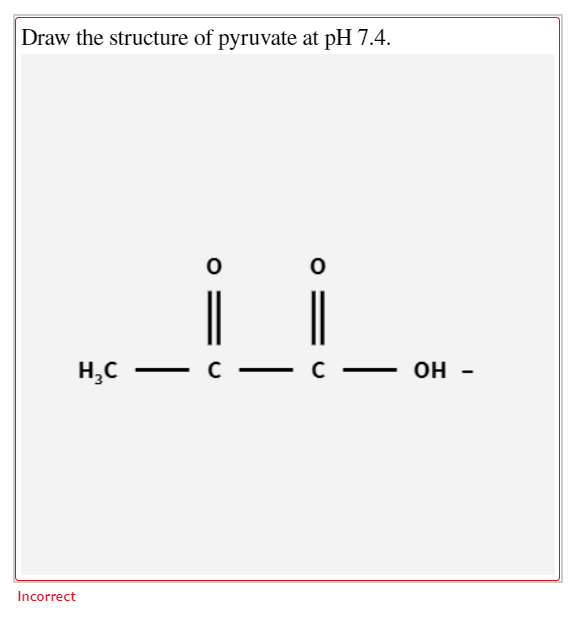
74 250 log pyruvatepyruvic acid 45log pyruvatepyruvic acid The antilog of 45 32 105 pyruvatepyruvic acid. Glucose ADP Pi NAD rar pyruvate ATP NADH Draw the structure of pyruvate showing its appropriate structure at pH 74. You can use a pen or possibly a dotting Instrument to have this consequence.
Draw the structure of the peptide LCYRAIDCG as it would exist at pH 65 under oxidizing conditions. 74 386 log lactatelactic acid 354 log lactatelactic acid The antilog of 354 35 103 lactatelactic acid. Glucose a ADP b P.
The molecule 2-propenoic acid has the structure. Select the structure of pyruvate showing its appropriate structure at pH 74. Glucose ADP Pi NAD rar pyruvate ATP NADH Draw the structure of pyruvate showing its appropriate structure at pH 74.
O O HC CC OH Incorrect. Draw the structure of pyruvate at pH 74. It is used for making acrylic polymers such as those that make artificial.
If the glucose molecule was labeled with 14C at the C-3 which carbon atom in. Pyruvate predominates in the cell at pH 74. Draw the structure of pyruvate showing its appropriate structure at pH 74.
O O HC CC OH. Select the structure of pyruvate showing its appropriate structure at pH 74. EH O-NA- Label the N-terminus of the peptide you drew in 3B above with a V.
Select the carbon atom of pyruvate that was originally the anomeric carbon aldehyde carbon in the open-chain form of glucose. The structure should have a carbon highlighted in green and bold that corresponds to the original 14C labeled C-3 of glucose. Label the C-terminus of the peptide you drew in 3B above with a Calculate the net charge of LCYRAIDG at pH 15.
Select the structure of pyruvate showing its appropriate structure at pHpH 74. The pK values for carboxylic acid groups are typically in the 2-3 range. The structure should have a carbon highlighted in green and bold that corresponds to the original anomeric carbon aldehyde carbon in the openchain form of glucose.
Draw the structure of pyruvate at pH 74. Up to 256 cash back If no coefficient is needed leave the answer blank. Draw the structure for each amino acid.
Draw the structure for each amino acid. Add coefficients to the reaction summary to show the net results of glycolysis. And the common name methacrylic acid.
Section 42 it actually exists primarily as pyruvate ion at physiological pH pH 74. Be sure to draw any disulfide bonds that could form. A tool that draws peptide primary structure and calculates theoretical peptide properties.
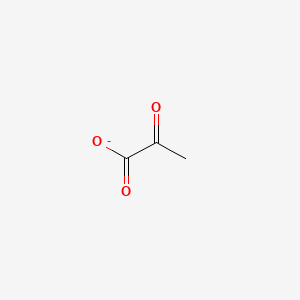
A Rank the six atoms listed in order of increasing electronegativity. Identify an amino acid whose side chain contains a. The chemical formula for pyruvic acid is C 3 H 4 O 3 and for its deprotonated form is C 3 H 3 O 3.
Or Butanoic acid butyric acid was first isolated from rancid butter from the Latin butyrum. C NAD x pyruvate y ATP z NADH You do not need to add the water and hydrogen ions necessary to balance the overall reaction. This nail art is great for the spring and summer.
The activities of the enzyme components of the pyruvate dehydrogenase complex are affected to different extents by changes in ionic strength and pH. Build five dots so which they hook up. Menu items go here.
Pyruvate Structure This molecule is the conjugate base of pyruvic acid a three-carbon molecule containing a carboxylic acid group and a ketone functional group. Predicted data is generated using the ACDLabs Percepta Platform - PhysChem Module. The solid line through the data points represents the fit to a mono-exponential or first order loss.
Ala A Arg R Asn N Asp D Cys C Gln Q Glu E Gly G His H Ile I. Draw the structure of pyruvate at ph 7 4. Identify an amino acid whose side chain contains an a.
A 2-oxo monocarboxylic acid anion that is the conjugate base of pyruvic acid arising from deprotonation of the carboxy group.

Add Coefficients To The Reaction Summary To Show The Net Results Of Glycolysis Glucose A Adp B Brainly Com

Solved 48 The Structure Of Pyruvic Acid Is Shown Below W Chegg Com
Solved Add Coefficients To The Reaction Summary To Show The Chegg Com

Oneclass Pyruvate Is The End Product Of Glycolysis Its Further Metabolismdepends On The Organism An
Solved Add Coefficients To The Following Reaction Summary To Chegg Com

Solved Add Coefficients To The Following Reaction Summary To Chegg Com
0 comments
Post a Comment